10 Avian Genetics
Avian Genetics Lab Intro
Authors: L. Taylor and C. Isaak
The chukar is a game bird native to Asia and was first introduced into Idaho in 1933. Now, chukars are often hunted for their meat. Idaho Fish and Game requests that hunters remove the right side wing and drop it in a labeled container when hunting. The wings are generally waste products; many hunters only consume the breast meat. The wings dropped in the barrels are used to determine the rough age of the bird, but because chukars are monomorphic (animals in which males and females are indistinguishable in appearance), it is nearly impossible to determine the sex of the bird by examining the wing. This is where our lab gets to help!

Hunters often care about conservation efforts for the birds and may donate the birds they have harvested to Boise State researcher, Cat Isaak. These donated birds are taken to a dissection lab where a small portion of breast tissue is removed for DNA analysis. During these dissections, the birds are inspected, measured, and aged based on the appearance of the wing feathers. The bird’s crop is also removed. The crop is a small muscular pouch in the neck that stores food before it is digested. The crop’s contents can be analyzed to learn more about the individual’s diet. Often, grasses and seeds are found in the chukars’ crops. Additionally, their digestive tracts, ceca, and gizzard can tell us a lot about the bird’s diet as well. The gizzard is a large muscular organ where most of the bird’s digestion occurs aided by rocks. Yes, rocks! Because birds have no teeth, they use rocks inside their gizzard to help grind up tough foods. Gizzards with thicker muscles may indicate that the bird had a poorer diet, while a shorter cecum may indicate a better diet. The contents of the crop and gizzard of a birds in good health can help us understand what types of diets are allowing the birds to thrive. If we determine what types of foods are being consumed by healthy chukars, we can inform land manager’s restoration efforts to plant native flora species that result in better condition birds. A high-quality diet indicates good soil and vegetation, leading to better body condition, improved winter survival, and higher breeding success. This boosts their population, providing more food for predators.
Why is the sex of the bird important?
Currently, IDFG collects population information with the use of the wing barrels or through observations provided by hunters or Wildlife Management Area staff who observe a group of birds that live together. There are no current methods for sex ratios for chukar. The wings from the barrel alone are not enough to determine sex, and sometimes during dissection the gonads are damaged, difficult to differentiate, or difficult to find, making DNA analysis necessary to confirm the gender of the birds. This data can help discover if males and females have different diets, the ratio of males and females in a population, and more. Knowing the ratio of males to females helps managers understand the reproductive potential of the population. A balanced sex ratio is essential for ensuring enough breeding females are present to sustain population growth or to maintain population numbers over time. In species managed for game (i.e. Alectoris chukar) it’s important to ensure that hunting practices do not disproportionately remove one sex. For example, overharvesting females could lead to reduced reproductive output, while altering natural sex ratios can have long-term impacts on population stability. Males and females may exhibit different behaviors or occupy different habitats. Detailed sex information allows managers to tailor habitat management plans and conservation efforts that account for these differences, ensuring both sexes have the conditions needed for survival and reproduction. Regularly collecting sex-specific data helps detect changes over time—whether due to environmental pressures, disease, or human influence. This information is key to adapting management practices in a timely manner to maintain healthy, sustainable populations.
Chelex Extraction Protocol – from Dr. Stephanie Galla
Pre-extraction steps (done by E. Meredith + C. Isaak)
1) Put tissues (breast tissue or blood) into 1.5mL microtubes. (Samples included 10 uL of blood; breast tissue chunk sizes varied)
2) Put 250µL of 10% chelex solution into tubes. Add 20 µL (20 mg/ml in water) of Proteinase K solution and incubate overnight at 55 °C in heat block.
Extraction steps for students:
1) Vortex samples for 5-10 seconds.
2) Add 250µL of 10% chelex solution into each microtube.
3) Place tubes in a preheated heat block (90°C) for 15 minutes. Put on safety goggles. Pop lids after the first 30 seconds to release pressure, and reclose them. Every 5 minutes, take samples off and vortex for 5-10 seconds.
4) After 15 minutes are up, vortex the samples one final time for 5-10 seconds and place immediately on ice for 5 minutes.
5) Spin the samples for 10 minutes at 10,200 x g in a centrifuge. Remove ~250µL of the supernatant and put into a freshly labeled microtube. Don’t pick up any of the resin, as it will inhibit PCR reactions.
6) Use for PCR and then store DNA at -20°C.
Avian PCR + Gel Protocol Guide
You and your partner will perform PCR on four different bird samples, along with a no-template control.
Primer set to amplify sex chromosomes:
CHD1F – TATCGTCAGTTTCCTTTTCAGGT
CHD1R – CCTTTTATTGATCCATCAAGCCT
We’re using slightly different PCR conditions than we have in the past, based upon the paper and the previous work by Dr. Galla. Fill in the blanks below determine the volumes you’ll need:
| Reagent | Final Concentration | Amount for a single 25 uL reaction | Total amount needed for all reactions |
| 5X Green GoTaq Buffer | 1X | ? | |
| 10 mM dNTPs | 200 µM | 0.5 uL | |
| 25 mM MgCl2 | 1.0 mM | ? | |
| 10 µM Forward Primer | 1 uL | ||
| 10 µM Reverse Primer | 1 uL | ||
| Taq DNA Polymerase (5 units/uL)
(Can dilute if necessary) |
1 UNIT per 25 µl PCR | ? | |
| Nuclease-free water | to 25 µl final volume | ? | |
| Template DNA | 1 uL DNA per 25 uL reaction |
We’ll be performing a different PCR technique called TOUCHDOWN PCR, which gradually decreases the annealing temp in each cycle.
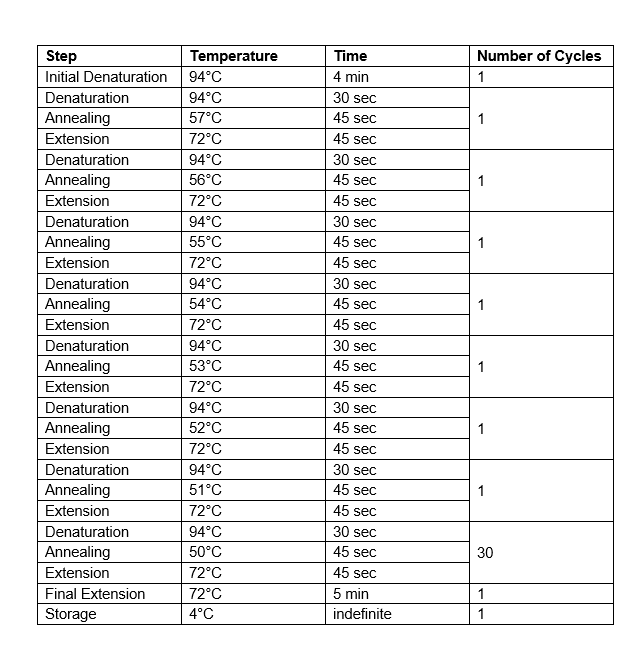
Table by George Harrentsian. Thanks George!
Gel guidelines:
Pour a 2% agarose gel with 1X TBE as your buffer. Run your gels for at least an hour at 100V to try to resolve potentially similarly sized DNA amplicons.
