8 Intro to protein analysis of muscles
Intro to muscle protein analysis
In this lab, we will examine the proteins found in muscle tissues. Muscle tissue consists of many different proteins with specialized functions, but the contraction of muscle results primarily from the interaction of two predominant proteins, actin and myosin. Actin and myosin form muscle fibers, the biochemical machinery that causes muscles to contract. These two proteins make up the structure and function of muscle that is common to all animals.

The genes for actin and myosin are members of gene families that encode proteins that enable movement. Other proteins associated with muscle have known, unknown, or speculated functions and may vary in their occurrence among different species. The variations in an organism’s proteins are the results of random DNA mutations within the encoding genes which have occurred over thousands to millions of years. Some mutations may result in a change in a protein, which can be beneficial, neutral, or detrimental to an organism’s ability to survive and reproduce.
How will you see a protein molecule?
In this investigation, you will first use high-resolution polyacrylamide gel electrophoresis (PAGE) to display the various proteins in the muscle tissue of different seafood items and perform molecular weight determinations. By the displays of the proteins assortments in the different seafood items, you can test the hypothesis that proteins are indicators of evolutionary relatedness.
You will use a combination of a detergent and heat to extract and denature (disrupt) the proteins in several fish muscle samples. The ionic detergent, sodium dodecyl sulfate (SDS), coats dissolved proteins and polypeptides with negative charges. The SDS-coated proteins then all move toward the positive electrode (anode) but at different rates depending on their sizes. When the functional proteins are coated with SDS and heated, they lose their three-dimensional structure and take on a net negative charge. Bigger polypeptides are coated with more molecules of SDS so the ratio of a protein’s molecular weight to its charge is approximately the same for all proteins. This means that size becomes the determinant of mobility through the gel.

The image above shows the denaturation of a protein (bottom-left) by sodium dodecyl sulphate (top-left). Detergent molecules coat the unfolded protein chain (right), which adopts a rod-like shape as a consequence of electrostatic repulsion. Negative charges contributed by SDS dominate the total charge of the aggregate and are used at the driving force in SDS gel electrophoresis. Image by Fdardel, CC-BY-SA.
Many large proteins are made of smaller protein subunits. These polypeptides are held together by bonds between sulfur atoms in the amino acids. Certain chemical treatments break these disulfide bridges and release the separate polypeptide units. We’ll be using a reducing agent (2-Mercaptoethanol (also called β-mercaptoethanol or BME) to accomplish this. Thus, one functional, native protein can give rise to several smaller polypeptides. Myosin, for example, is a complex of 2 heavy protein chains and 4 light chains. The light chains are of two different sizes, so a purified myosin sample will form 3 separate bands when treated appropriately prior to electrophoresis.
Determining the size of proteins via electrophoresis
To analyze proteins, we will use polyacrylamide instead of agarose gels. The gel matrix formed by polyacrylamide is much tighter and able to resolve much smaller molecules than agarose gels. Polyacrylamide gels have pore sizes similar to the size of proteins. DNA molecules are orders of magnitude larger than proteins and for nucleic acids, agarose is usually the preferred gel medium. However, when separating very small fragments of DNA, such as during DNA sequencing, polyacrylamide is the gel matrix of choice. Polyacrylamide gels are much thinner and more fragile than agarose gels, so be very careful when you handle them!
Untreated or native proteins will migrate in a gel at rates based on both their electrical charges and their masses. If we equalize charge-to-mass ratios (charge densities) of all protein molecules, mass becomes the only factor determining the migration rate of each protein. This is accomplished by treating the proteins with the ionic detergent SDS, which is present in both the gel running buffer and the sample loading buffer. This technique is called SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Protein size is measured in daltons, a measure of molecular weight. One Dalton is defined as the mass of a hydrogen atom, which is 1.66 x 10 -24 gram. Most proteins have masses on the order of thousands of daltons, so the term kilodalton (kD) is often used to describe protein molecular weight. Given that the average weight of an amino acid is 100 daltons, the number of amino acids in a protein can be approximated from its molecular weight.
What can proteins tell you about evolution?
The banding patterns on your gel reveal information about the protein composition of muscle from your seafood samples. Proteins are synthesized according to the genes of an organism’s DNA. Since closely related organisms share similar DNA sequences, the proteins encoded by their DNA should be very similar as well, and similar protein compositions should be reflected in banding patterns. Different branches of related organisms separated at different evolutionary times. The further apart species are on the tree, the less related they are. Mollusks and arthropods diverged from one another before the emergence of chordates, animals with backbones, very early in evolutionary time. These animals are only distantly related to fish, birds, reptiles, mammals and amphibians, which are more closely related to each other. We will compare the information we can obtain from protein samples with the information we can obtain from analysis of the amino acid sequences of light chain myosin muscle proteins made in these organisms.
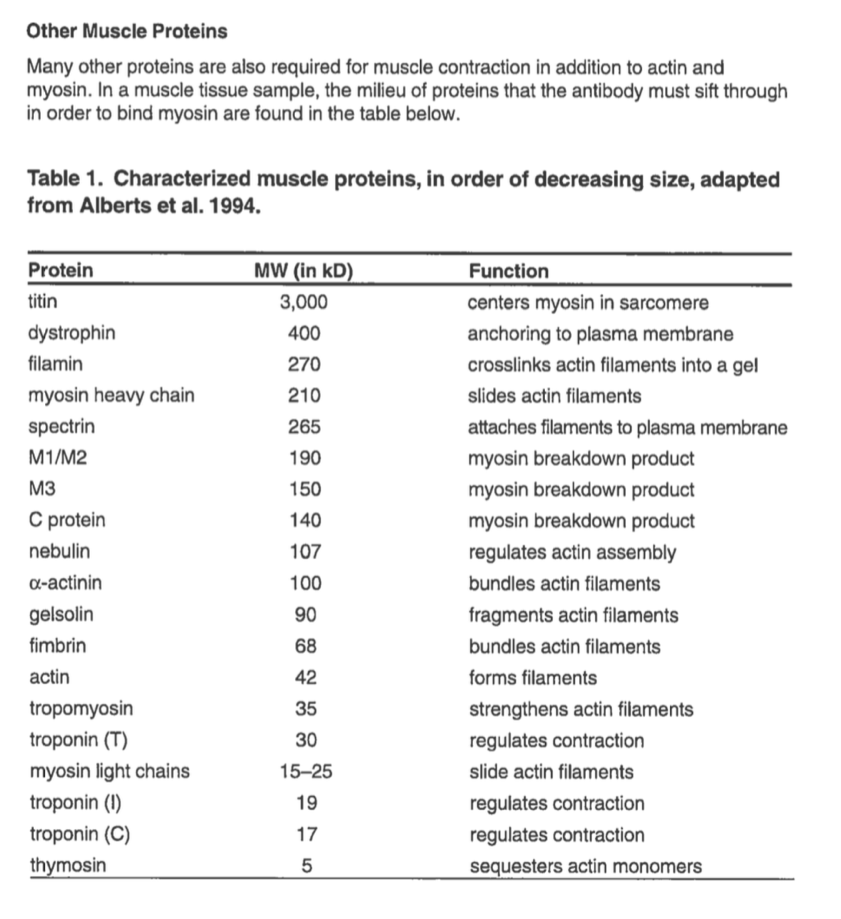
Here is a list of common muscle proteins. You can use this list to analyze your protein gels.