15 Preparation for Sanger Sequencing
Preparing your PCR products for DNA sequencing
- We will submit our samples for sequencing in PCR strip tubes. To avoid contamination, you will each prep your sample in a sterile 1.7 mL mL tube, label it appropriately, and we’ll transfer your sample to a PCR tube for sequencing.
- The sequencing primer is diluted to 5 µM (pmol/µl) using water. You need 5 µl of primer for each 15 uL sequencing reaction. Only one primer is used in a sequencing reaction. You are just making a copy of one strand of DNA.
- Determine the concentration of your PCR product (template) with a Nanodrop.
- Notice that the size of your amplicon impacts how much DNA is needed for sequencing. Use your DNA concentration to calculate the volume of DNA you need to achieve the necessary ng of DNA.
- The final volume of your diluted DNA will be 10 µl, so you’ll add water to dilute your DNA based upon your calculations.
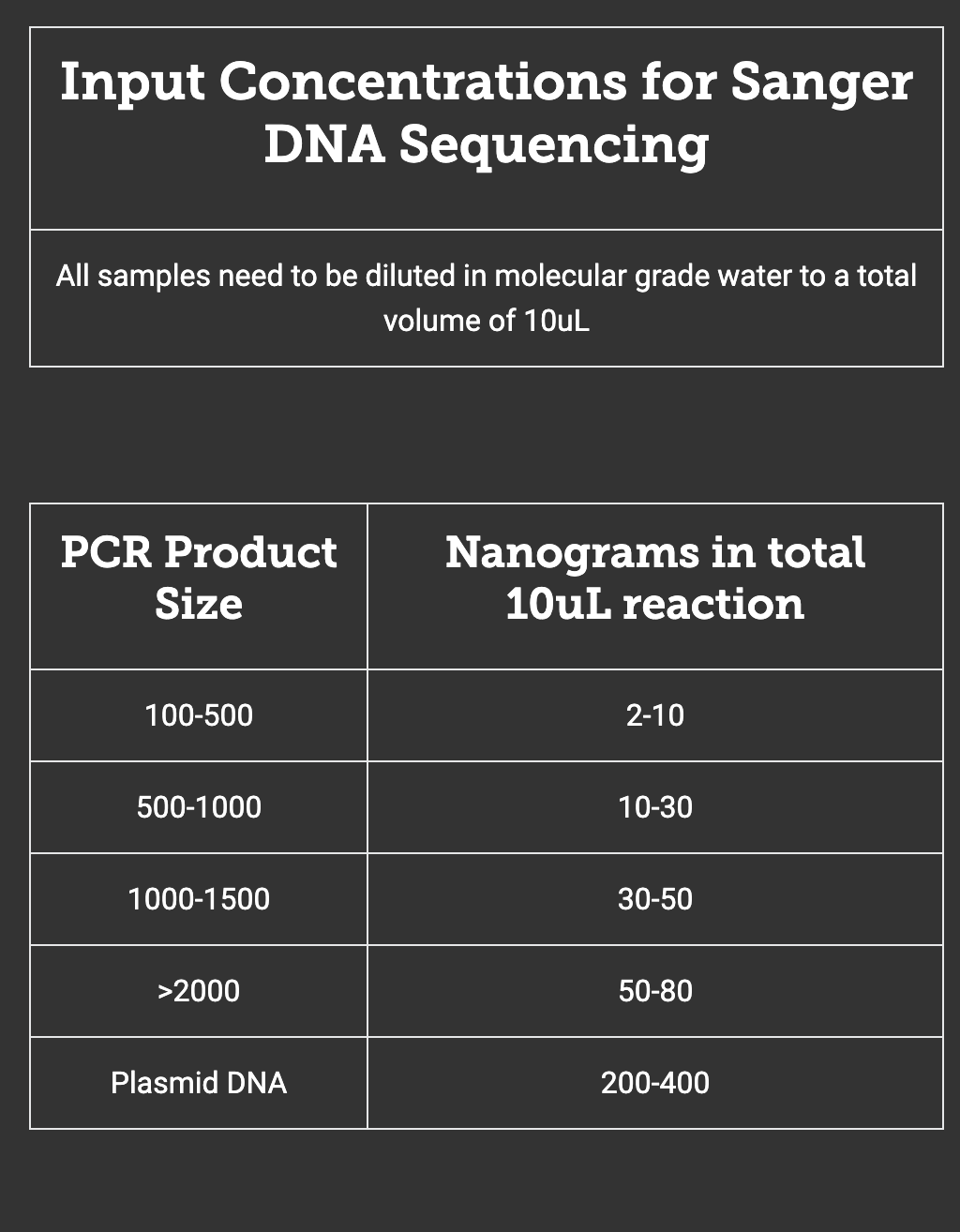
Math example: Imagine your purified PCR amplicon is 500 bp long. Your Nanodrop results shows this DNA is at 8 ng/uL. From the table below, you can see that this means you need to add 10 ng to your sequencing reaction. So, set up the math, to solve for x—the volume of DNA that you’ll dilute in water to yield your 10 µl diluted DNA.
8 ng/uL * x uL = 10 ng ……. so, x = 1.25 uL of DNA.
The rest of the volume is water, so we also need to add 10-1.25= 8.75 uL water
Note: if your math reveals you need to add < 0.5 uL of your DNA, you need to do a 1:10 dilution of your DNA first and then redo your math since you cannot accurately pipette volumes < 0.5.
- Mix together 5 uL of sequencing primer with the 10 uL of diluted DNA in a microcentrifuge tube.
- Ensure you have labeled your tube with the unique identifier you were provided.
For the mtDNA experiment, our sequencing primer is:
CCA TTA GCA CCC AAA GCT AAG ATT
