17 Protein sample prep and SDS-PAGE
Preparing samples for protein gel electrophoresis:
Laemmli sample buffer is used to prepare proteins for SDS-PAGE with Tris-glycine-SDS running buffer. You will use already prepared sample buffer that contains:
- 2X Laemmli sample buffer
- 65.8 mM Tris-HCl, pH 6.8
- 26.3% (w/v) glycerol
- 2.1% SDS
- 0.01% bromophenol blue
A reducing agent has been added for you just prior to use: 50 uL of 2-mercaptoethanol per 950 uL sample buffer
Why add a reducing agent?
Cleaving intermolecular (between subunits) disulfide bonds allows the subunits of a protein to separate independently on SDS-PAGE. Cleaving intramolecular (within subunit) disulfide bonds allows the subunits to become completely denatured so that each peptide migrates according to its length.
Prepare your samples to be loaded onto a gel:
- Each table will collect a sample from each type of seafood.
- Cut a piece of muscle tissue that is 0.25 cm x 0.25 cm x 0.25 cm3 (About the size of a tic-tac).
- Weigh each sample and transfer it to a microfuge tube.
- Based on the weights, calculate how much sample buffer you will add to each muscle sample so that the relative proportion of sample buffer:muscle is the same for all tubes. Use 250 uL of Laemmli sample buffer (containing the reducing agent) for your sample with the median weight and scale the remaining sample buffer volumes accordingly.
- Flick each tube to agitate the tissue ~ 15 times.
- Incubate at room temperature for 5 min. Flick again.
- Pipette the liquid from the tube into a new microfuge tube. DO NOT transfer any chunks of muscle. This liquid is your PROTEIN EXTRACT.
- Put on a boiling cap and heat sample at 5 min at 95 °C to denature the proteins.
- After boiling step, spin down tube before opening lid.
- Use samples immediately or store at -20 C for future use.
Protein Gel electrophoresis
You can pour these gels yourself or buy them from a company. Pouring gels takes a while, and several of the reagents are potentially dangerous (For example, the acrylamide is a suspected carcinogen), so we are using “pre-cast gels”.
- If frozen, reheat protein extracts at 95 °C for 2-5 minutes to re-dissolve the SDS.
- Prepare 750 1X TGS Running buffer (Tris-glycine-SDS). You will need about 700 mL per gel box.
- Take your precast gel out of its package. Make sure that you remove the strip of green tape from the bottom of the gel.
- Lock your gel in place within the clamping frame with the SHORT place facing inwards. If you only run one gel, you will use a “Buffer Dam” as its partner in the gel box.
- Fill the inner chamber with buffer. Make sure that the buffer is above the top of the smaller plate. After filling the inner chamber, wait a couple minutes to check for leaks before filling the outer chamber. Carefully pull out the comb, going as slowly as possible to avoid damaging the wells.
- After you’ve checked for leaks, fill OUTER CHAMBER up to the correct line (2 gel line)
- Load your samples into the gel. Use a 10 uL pipette and go very slowly. You should see your sample fall neatly into the bottom of each well.
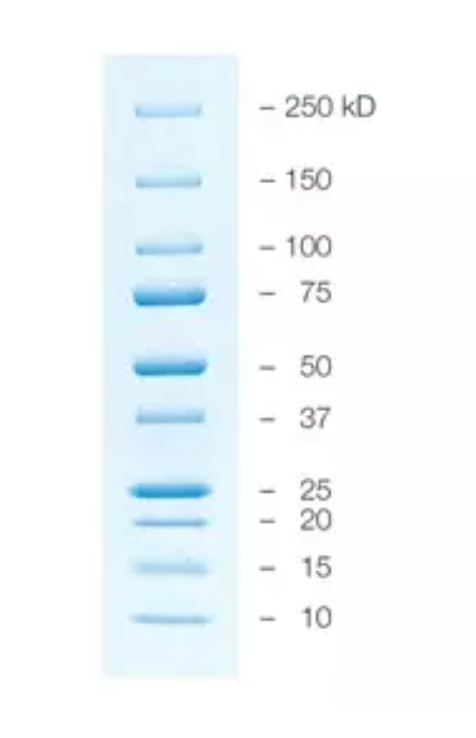
- For your ladder, you will load 5 uL of the Precision Plus Protein Kaleidoscope prestained protein standard.
- For your samples, load 10 uL per well.
- Place the lid on your gel box (CHECK ORIENTATION) and set voltage to 200 V. Your gel should run for ~30 minutes. Watch the pre-stained proteins separate and monitor the blue dye to assess where your proteins are running.
- Remove your gel from the box and discard buffer (down drain).
- To stain your gel, pry apart the plastic gel plates. Transfer your gel to a tray containing diH2O. Rinse gel 3 times with water for 5 minutes each wash. (DO NOT LET YOUR GEL FALL OUT).
- Stain gels for 40 min in 30 mL of Bio-Safe Coomassie Stain with gentle shaking (stain until you can see bands). Coomassie blue dyes are a family of dyes commonly used to stain proteins in SDS-PAGE gels. The gels are soaked in dye, and excess stain is then eluted with a solvent (“destaining”). This treatment allows the visualization of proteins as blue bands on a clear background.
- Rinse gel in 200 mL of diH2O, changing the water every 15 minutes. Rinse gel and continue destaining until the background is reduced and you can see clear bands. You can destain overnight if necessary.
Precision Plus Standards Protein ladder
Protocol:
- Keep ladder on ice.
- Load 5 uL ladder per lane. The loading buffer is already supplied in the ladder mixture.
- Do not boil the protein ladder before loading it onto the gel.