20 Worm RNAi protocol
RNAi in C. elegans protocol
Week 1: Day 1
Objective 1: Prepare nematode growth medium (NGM)
Each person is responsible for pouring plates. Make sure your bench is disinfected and that you minimize contamination.
Half the class will pour NGM-lite plates, which is the normal growth medium for C. elegans
- When the medium bottle can be held comfortably (~55 C ), this medium requires the addition of cholesterol. Using sterile technique, one vial of cholesterol must be added to the medium before pouring plates.
- Pour small 60 mM plates so that medium entirely covers surface of plate—Each bottle contains enough media to pour at least 24 plates.
The other half of the class will pour NGM-lite with AMP and IPTG plates, which is the growth medium for RNAi E. coli strains that must be induced to make dsRNA.
- When the medium bottle can be held comfortably (~55 C), this medium requires the addition of cholesterol. Using sterile technique, add one vial of cholesterol to the medium before pouring plates.
- Add 3 mL of ampicillin at 10 mg/mL (100X stock)
- Add 120 uL of IPTG (to induce T7 expression)
- Pour small 60 mM plates so that medium entirely covers surface of plate—Each bottle contains enough media to pour at least 24 plates.
- Let your plates dry on your bench until you are leaving lab.
- Stack and invert, and store in air-tight bag in the fridge until Day 3 of the experiment.
Objective 2: Each group (of four) will start the three following E. coli cultures that we will feed to the worms.
- OP50: Control E. coli (do not contain siRNA genes)
- bli-1 RNAi E. coli
- dpy-11 RNAi E. coli
Use aseptic technique in all of your work. Clean glass bottles with ethanol (including the threads) and do your best to minimize potential contamination of your media.
- The OP50 bacteria are grown in LB broth (with no antibiotic).
- Pipette 1 mL of LB broth into a sterile culture tube
- Use a sterile loop to scrape a small amount of bacteria from the slant culture. If you can see a tiny amount of bacteria on the loop, you have transferred enough. Aim for the amount in ~1 small colony.
- Swirl the loop into the LB broth.
2. The RNAi strains are grown in LB with ampicillin (100 ug/mL final concentration)
- Pipette 1 mL of LB broth WITH AMP into a sterile culture tube.
- Use a sterile loop to scrape a small amount of bacteria from the slant culture. If you can see a tiny amount of bacteria on the loop, you have transferred enough. Aim for the amount in ~1 small colony.
- Swirl the loop into the LB broth.
- Do not fully seal the lids, so that oxygen can reach the bacteria. Incubate the tubes in incubator at 37 C for 48 hrs without shaking.
Don’t forget to disinfect your bench, wash your hands, and make sure anything that had contact with bacteria will get autoclaved or disinfected.
Week 1: Day 2. Seed worm plates with bacterial cultures.
Reminder: Make sure you label each plate with which bacteria you are spreading.
- Each group of two will seed two NGM-lite plates with OP50 bacteria. Add 50 uL in the center of each NGM lite plate and gently spread with a small, sterile glass rod. The bacteria should cover most of the plate, but do not let the culture reach the side of the plate, or the worms can crawl out and die from water loss.
- Each group of two will seed one NGM lite/AMP+IPTG plate with bli-1 RNAi bacteria, and one NGM lite/AMP+IPTG plate with dpy-11 bacteria. Cover the surface of the plate with the feeding strain by adding 50 uL culture to the center of each plate and gently spread with a small, sterile glass rod.
- Clean up correctly and disinfect your bench.
- Incubate the plates overnight at room temperature with the plate face-up (lid on top; not inverted).
- After this incubation, the plates are stored at 4 °C until needed. The plates can last for ~ 1 month in the fridge.
Week 2, Day 1: Add worms to the NGM lite plates (Worm “chunking”)
- Sterilize a metal spatula by dipping it in ethanol and igniting with a lighter or bunsen burner.
- Use a sterilized spatula to cut a 1 cm square chunk of agar with worms on it from the wildtype starter plate (worms that have not been treated with anything)
- Place the chunk, face down, on ONE bacteria covered NGM lite plate (Placing the chunk face-down means the worms crawl quickly onto the plate). Verify with a microscope that you have transferred worms.
- Incubate the plate, lid-side down (INVERTED) for 2-3 days at ~20 °C. Clean out your drawer with ethanol. You will store your worms in your clean drawer (they like cool, dark places).
Week 2, Day 2: Induce RNAi by Feeding
- Transfer five L4 stage worms to an OP50 plate labeled “wild-type.” TIP: The point of the toothpick works best to grab the worms. Try scraping the surface of the agar instead of stabbing into it.
- Make sure you do not kill, squish, mame, or injure your worms during transfer. After moving them, examine them under the scope to make sure they are still healthy. Pick off any eggs or young larvae that may have been accidentally transferred.
- Repeat steps 1-2 and transfer five L4 worms to the bli-1 and dpy-11 RNAi plates.
- Incubate the worms, lid side down, at 20° C (room temperature).
How to pick an L4 worm:
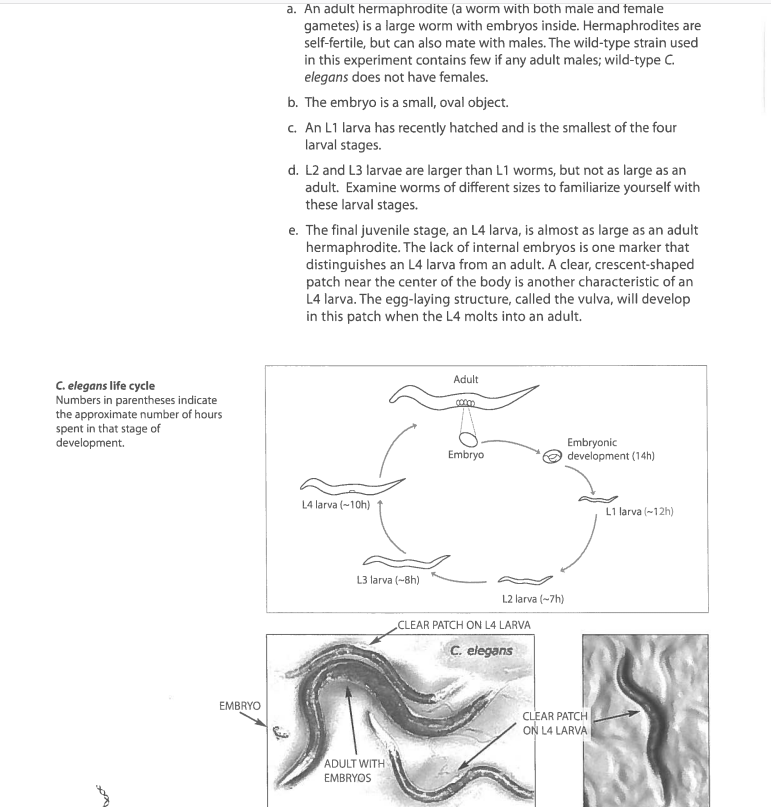
Week 3 Day 1: Assessing the worm phenotype
- Observe your control plate of worms first. Make notes on whether most of the worms in each of the developmental phases listed below appear healthy and are motile. Describe your observations in words, and make rough estimates of what percentage of worms are in each of the following phases of growth.
- Then, observe the RNAi plates and note the differences you observe in the morphology or behavior of these worms compared to the worms fed control bacteria. Fill in the table below as you make your observations.
- On each RNAi plate, count the number of ADULT worms that appear normal and those that show a noticeable phenotype. As you count a worm, remove it from the plate or squish it so that you do not re-count it in your analysis. Each person must count at least 30 worms on each plate to get reliable data. As you count, do not discuss your results with your partner so that you do not bias your results.
- Calculate the percent of worms that were affected out of the total number of worms that your group has scored.
Conclusions: You should address the following questions in your conclusions sections:
- Which worms were affected? Adults? L1-L3? L4? Was the most noticeable phenotype different than what you expected? Did you results support your hypothesis?
- Are your results consistent with what you determined about when and where these genes are expressed in the worms? (Remember when you learned about these genes on wormbase!)
- What would you do next?
- What did you learn from doing RNAi? What do these experiments tell us about the importance/function of these genes?
