101 17.6 Corrosion
Learning Objectives
- Define corrosion
- List some of the methods used to prevent or slow corrosion
Corrosion is usually defined as the degradation of metals due to an electrochemical process. The formation of rust on iron, tarnish on silver, and the blue-green patina that develops on copper are all examples of corrosion. The total cost of corrosion in the United States is significant, with estimates in excess of half a trillion dollars a year.
Statue of Liberty: Changing Colors
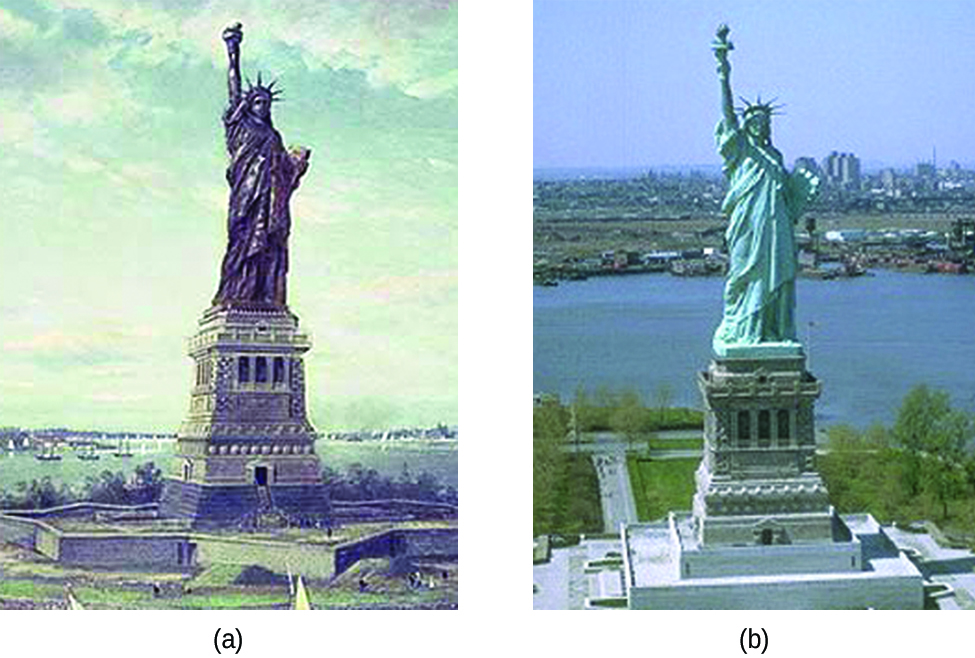
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green color (Figure 1). When this statue was first delivered from France, its appearance was not green. It was brown, the color of its copper “skin.” So how did the Statue of Liberty change colors? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Copper metal is oxidized to copper(I) oxide (Cu2O), which is red, and then to copper(II) oxide, which is black
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin. The formation of the protective layer is a form of passivation, which is discussed further in a later chapter.
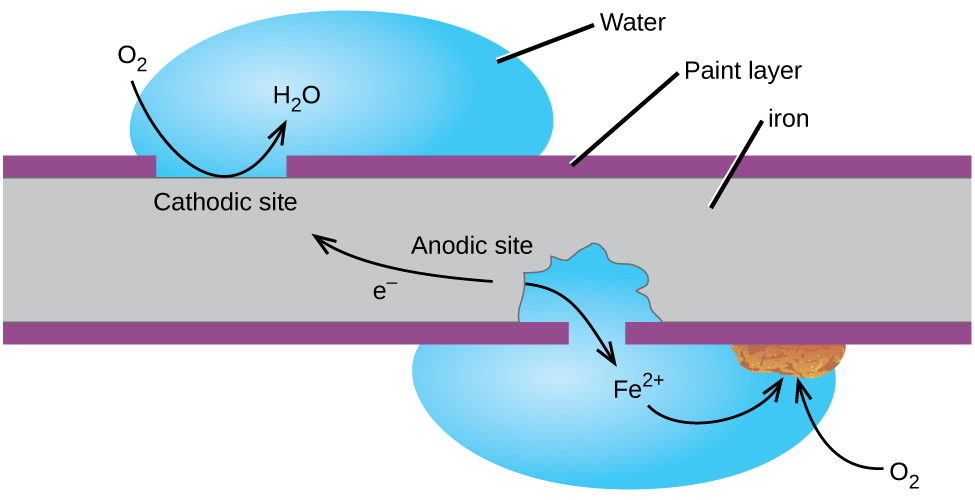
Perhaps the most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following (Figure 2). Once exposed to the atmosphere, iron rapidly oxidizes.
The electrons reduce oxygen in the air in acidic solutions.
What we call rust is hydrated iron(III) oxide, which forms when iron(II) ions react further with oxygen.
The number of water molecules is variable, so it is represented by x. Unlike the patina on copper, the formation of rust does not create a protective layer and so corrosion of the iron continues as the rust flakes off and exposes fresh iron to the atmosphere.
One way to keep iron from corroding is to keep it painted. The layer of paint prevents the water and oxygen necessary for rust formation from coming into contact with the iron. As long as the paint remains intact, the iron is protected from corrosion.
Other strategies include alloying the iron with other metals. For example, stainless steel is mostly iron with a bit of chromium. The chromium tends to collect near the surface, where it forms an oxide layer that protects the iron.
Zinc-plated or galvanized iron uses a different strategy. Zinc is more easily oxidized than iron because zinc has a lower reduction potential. Since zinc has a lower reduction potential, it is a more active metal. Thus, even if the zinc coating is scratched, the zinc will still oxidize before the iron. This suggests that this approach should work with other active metals.
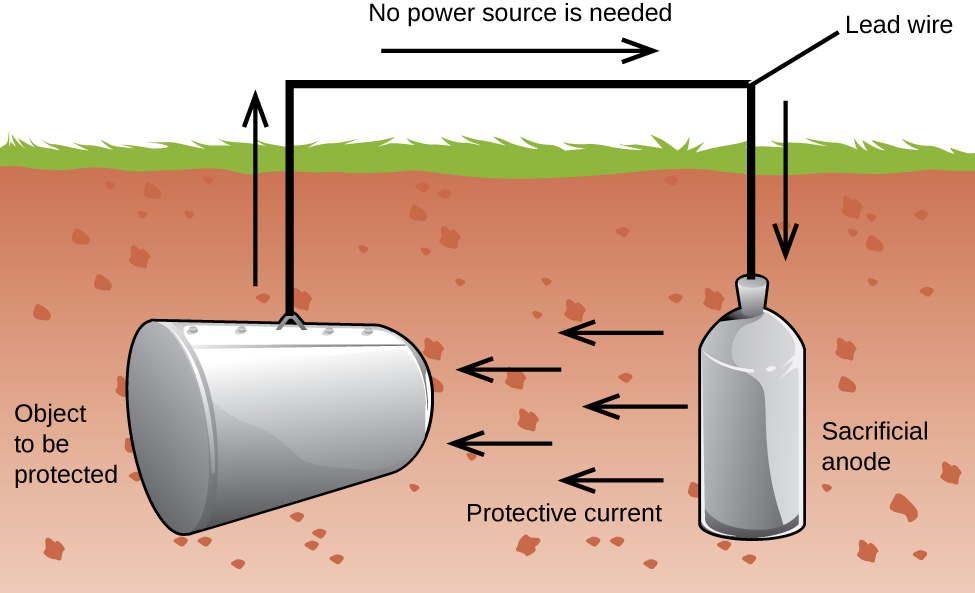
Another important way to protect metal is to make it the cathode in a galvanic cell. This is cathodic protection and can be used for metals other than just iron. For example, the rusting of underground iron storage tanks and pipes can be prevented or greatly reduced by connecting them to a more active metal such as zinc or magnesium (Figure 3). This is also used to protect the metal parts in water heaters. The more active metals (lower reduction potential) are called sacrificial anodes because as they get used up as they corrode (oxidize) at the anode. The metal being protected serves as the cathode, and so does not oxidize (corrode). When the anodes are properly monitored and periodically replaced, the useful lifetime of the iron storage tank can be greatly extended.
Key Concepts and Summary
Corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the effects of, or preventing, corrosion. Some metals, such as aluminum and copper, produce a protective layer when they corrode in air. The thin layer that forms on the surface of the metal prevents oxygen from coming into contact with more of the metal atoms and thus “protects” the remaining metal from further corrosion. Iron corrodes (forms rust) when exposed to water and oxygen. The rust that forms on iron metal flakes off, exposing fresh metal, which also corrodes. One way to prevent, or slow, corrosion is by coating the metal. Coating prevents water and oxygen from contacting the metal. Paint or other coatings will slow corrosion, but they are not effective once scratched. Zinc-plated or galvanized iron exploits the fact that zinc is more likely to oxidize than iron. As long as the coating remains, even if scratched, the zinc will oxidize before the iron. Another method for protecting metals is cathodic protection. In this method, an easily oxidized and inexpensive metal, often zinc or magnesium (the sacrificial anode), is electrically connected to the metal that must be protected. The more active metal is the sacrificial anode, and is the anode in a galvanic cell. The “protected” metal is the cathode, and remains unoxidized. One advantage of cathodic protection is that the sacrificial anode can be monitored and replaced if needed.
Chemistry End of Chapter Exercises
- Which member of each pair of metals is more likely to corrode (oxidize)?
(a) Mg or Ca
(b) Au or Hg
(c) Fe or Zn
(d) Ag or Pt
- Consider the following metals: Ag, Au, Mg, Ni, and Zn. Which of these metals could be used as a sacrificial anode in the cathodic protection of an underground steel storage tank? Steel is mostly iron, so use −0.447 V as the standard reduction potential for steel.
- Aluminum [latex](E_{\text{Al}^{3+}/\text{Al}}^{\circ} = -2.07\;\text{V})[/latex] is more easily oxidized than iron [latex](E_{\text{Fe}^{3+}/\text{Fe}}^{\circ} = -0.477\;\text{V})[/latex], and yet when both are exposed to the environment, untreated aluminum has very good corrosion resistance while the corrosion resistance of untreated iron is poor. Explain this observation.
- If a sample of iron and a sample of zinc come into contact, the zinc corrodes but the iron does not. If a sample of iron comes into contact with a sample of copper, the iron corrodes but the copper does not. Explain this phenomenon.
- Suppose you have three different metals, A, B, and C. When metals A and B come into contact, B corrodes and A does not corrode. When metals A and C come into contact, A corrodes and C does not corrode. Based on this information, which metal corrodes and which metal does not corrode when B and C come into contact?
- Why would a sacrificial anode made of lithium metal be a bad choice despite its [latex]E_{\text{Li}^{+}/\text{Li}}^{\circ} = -3.04\;\text{V}[/latex], which appears to be able to protect all the other metals listed in the standard reduction potential table?
Glossary
- cathodic protection
- method of protecting metal by using a sacrificial anode and effectively making the metal that needs protecting the cathode, thus preventing its oxidation
- corrosion
- degradation of metal through an electrochemical process
- galvanized iron
- method for protecting iron by covering it with zinc, which will oxidize before the iron; zinc-plated iron
- sacrificial anode
- more active, inexpensive metal used as the anode in cathodic protection; frequently made from magnesium or zinc
Solutions
Answers to Chemistry End of Chapter Exercises
2. Mg and Zn
4. Both examples involve cathodic protection. The (sacrificial) anode is the metal that corrodes (oxidizes or reacts). In the case of iron (−0.447 V) and zinc (−0.7618 V), zinc has a more negative standard reduction potential and so serves as the anode. In the case of iron and copper (0.34 V), iron has the smaller standard reduction potential and so corrodes (serves as the anode).
6. While the reduction potential of lithium would make it capable of protecting the other metals, this high potential is also indicative of how reactive lithium is; it would have a spontaneous reaction with most substances. This means that the lithium would react quickly with other substances, even those that would not oxidize the metal it is attempting to protect. Reactivity like this means the sacrificial anode would be depleted rapidly and need to be replaced frequently. (Optional additional reason: fire hazard in the presence of water.)

