6 Preparing Your Research Proposal
More often than not, there will be a few steps that you’ll have to take before you can start gathering and analyzing data in pursuit of an answer to your research question. Preparing a research proposal is a milestone in any research project and is often required by sponsoring institutions in order to transition from ‘the ‘planning’ phase to the ‘doing’ phase. So why, you might ask, are we talking about this step in phase III, ‘writing’? That’s a great question and it has to do, primarily, with the order of thought and the information that must be included in a research proposal. In this chapter, we’ll cover the basic requirements of most research proposals and address the requirements and responsibilities of a researcher.
Chapter 6: Learning Objectives
Before you prepare to implement your research methodology, it is likely that you’ll need to gain approval to continue. As we explore the development of the research proposal, you’ll be able to:
- Describe the individual elements of a research proposal
- Delineate between the rationale and implementation portions of a research proposal
- Discuss the ethical tenets which govern researchers
- Define the purpose of an institutional review board
- Compare categories of institutional review board applications
What is a research proposal?
A research proposal can be thought of as the general blueprint for a proposed research project. There are very few instances wherein research projects can be pursued without support of a sponsoring institution. That is, a healthcare system, hospital, or academic institution. To receive support from a sponsoring institution, a researcher must articulate a clear plan for their research process to include:
- An overview of the literature which supports the investigation
- A statement of the problem
- A statement of purpose
- A hypothesis or central question
- An overview of how participants will be identified, selected, contacted or data will be identified, analyzed, and protected
- An overview of the proposed methodology (i.e. approach to the study)
- An acknowledgment that participants, data, and results will be treated ethically throughout the study
- A timeline for the project
As Crawford, Burkholder, and Cox (2020) describe, these items can be split into separate portions of a research proposal, the rationale (i.e. Whye) and implementation (i.e. How).
Rationale
As we discussed in previous chapters, developing a robust rationale for your research will help guide the entire research process. The introduction to your research proposal should include a general description of why the research should be conducted. Aside from your general interest, the introduction to the research should be firmly rooted in the available evidence which, first identifies a problem; second, identifies a purpose for the pursuit of inquiry into the problem; and finally, articulates a clear and focused research question which addresses the gap in current knowledge on the topic.
Implementation
Outlining your plan for implementation is essential to gain approval to conduct your research. Equally important to developing a well-articulated rationale, the identification of a clear methodology for how you will implement your approach is an important component of a research proposal.
A plan of implementation can be presented in several ways. However, an inclusive plan should include the following elements (Crawford, Burkholder, & Cox, 2020):
- Design
- Methods
- How you will select participants or identify ‘what’ is included in your investigation
- How you will measure what you’re investigating
- What type of data you will collect and how
- How you will analyze the data
- Delineators
- Definitions
- Frame the terms that specify your investigation
- Assumptions
- Qualities of the study that are inherent to the study, but may be overlooked as obvious unless addressed
- Delimitations & Limitations
- Delimitations narrow the scope of the study regarding what it does not include. Limitations are an acknowledgement of the weaknesses of the study design or methodology (Spoiler: there are limitations to EVERY study).
- Significance
- How does your research impact the field of inquiry? Does it:
- Influence practice?
- Impact policy?
- Provide a foundation for future research?
- How does your research impact the field of inquiry? Does it:
- Definitions
We’ve spent a lot of time discussing how to identify a problem, a purpose, articulate a question, and identify a sample and the selection and implementation of an appropriate approach. Ethical considerations of the researcher is another essential topic for any researcher to cover. Here, we’ll provide a general overview of ethical considerations that are required of sponsoring institutions to ensure the ethical treatment of study participants and related data.
Ethics
As a clinician, you’re likely familiar with the tenets of bedside bioethics that guide clinical practice:
- Autonomy: The right to self-direction and control
- Beneficence: The intention to do ‘good’, or what is in the best interest of the patient
- Non-Maleficence: The goal to ‘do no harm’ in practicing
- Justice: The pursuit of fairness and equity
These basic tenets of care do not change much when viewed through the lens of a researcher. However, it is important to note the foundation upon which research ethics were built. In 1974, the National Research Act was drafted in response to blatant abuse of research methods such as the Tuskegee study and resulted in the establishment of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research.The ethical principles which guide researchers are derived outlined by the Belmont Report (HHS.gov) and include:
- Autonomy: Respect for a person to make personal choices and provisions and protections to be provided for participants belonging to vulnerable populations
- Beneficence: The intention to do what is morally right; to minimize risk and maximize benefits
- Justice: To promote equity among the treatment of individuals and groups
Researchers must address the ways in which they intent to uphold these principles in their proposed research project. Methods by which they do this include:
- Voluntary Informed Consent: Informed consent is a process which ensures that a participant is educated in terms that they can understand about the risks inherent to their participation. This process underscores respect through the provision of consent for a voluntary act (HHS.gov, n.d.)
- Avoidance of Harm: Avoidance of harm is related to the ethical tenet of beneficence and is the primary responsibility of the researcher
- Assessment of Risk: The common rule mandates that researchers ensure that the risk to potential participants in a research study are minimized and that the research cannot impose risk that outweighs the potential benefit of the outcomes.
- Right to Withdrawal: Participants must be made aware of their rights to withdraw from the study at any time, for any reason, without consequences.
- Responsibility to Terminate: The principle investigator has the responsibility to terminate the research intervention should it be made clear that the intervention has either a detrimental effect on participants or an overwhelmingly positive effect such that it would be unethical to continue the study.
Universal research practices which promote these principles must be included in a research proposal in order to conduct research at most institutions and are outlined in the Common Rule which regulates the functions of institutional review boards (IRBs).
Institutional Review Board
An IRB is a formally designated group which has been established to protect the rights and welfare of human subjects recruited to participate in research; specifically research conducted at, or supported by, a specific institution. Here it is important to understand what is meant by the terms ‘research’ and ‘human subjects’. In regards to the requirement of IRB review, the term research means a systematic investigation, development, testing and evaluation designed to develop or contribute to generalizable knowledge (University of Southern California, n.d.). Human subjects in relation to research refers to a living individual who’s information or biospecimens are used or analyzed to generate either identifiable private information or biospecimens for the purpose of generalizable information (University of Southern California, n.d.).
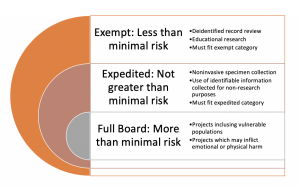
Although there are some details which will differ between organizations, there are general categories of human subject research which must be reviewed by an IRB. These classifications are designated by the degree of risk assumed by the participants and the ability of the researcher to mitigate those risks. Minimal risk is described by the federal regulations as the probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental, or psychological examination of healthy persons (Electronic Code of Federal Regulations, n.d). Generally, research proposals will fall into one of the following categories:
- Exempt: Exempt research poses no more than minimal risk to adult, non-vulnerable populations.
- Expedited: Research that poses no more than minimal risk to participants and fits into one of the expedited categories described in federal regulations 45 CFR 46.110 (HHS.gov)
- Full Board: Research that does not qualify for either exempt or expedited review and poses more than minimal risk to participants. This type of review requires the approval from a full membership of an IRB.
Projects that don’t need IRB approval
Projects which are not considered human subjects research are not required to be reviewed by an IRB. Quality improvement projects do not typically require formal IRB review. However, individual institutional requirements should be reviewed and followed; preferably, in the planning phase of your research project to ensure that the requirements of your specific review align with both your approach and your timeline.
Key Takeaways
- Research proposals can be split into two primary components: The rational and the plan of implementation
- The introduction of your research proposal should encompass a description of your problem, purpose, and research question
- The identification of your research approach should be firmly guided by the ethical tenets of autonomy, beneficence, and justice
- The researcher has an ethical responsibility to protect participants from risk
- An institutional review board is a formal board charged with reviewing risks associated with research projects
- There are differing levels of institutional review; assumption of risk is the primary factor in classifying level of IRB review
References
Crawford, L.M., Burkholder, G.J., Cox, K.A. (2020). Writing the Research Proposal. In G.J. Burkholder, K.A Cox, L.M. Crawford, and J.H. Hitchcock (Eds.), Research design and methods: An applied guide for the scholar-practitioner (pp. 309-334). Sage Publications
Electronic Code of Federal Regulations. (2020, August, 17). Protection of human subjects. Electronic Code of Federal Regulations. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#se45.1.46_1104
Health and Human Services. (2020, August, 14). The Belmont report. Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/index.html
University of Southern California. (2020, August, 17). Office for the protection of research subjects. University of Southern California. https://oprs.usc.edu/irb-review/types-of-irb-review/
The right to self direction and control
The intention to do 'good'
The intention to do no harm
Pursuit of fairness and equity
A systematic investigation
Living persons participating in research
Probability of harm that does not exceed that encountered in every day life
IRB classification for research projects that do not pose more than minimal risk to adult, non-vulnerable populations
Classification of IRB approval for research that does not pose more than minimal risk, but fits into federally regulated categories.
IRB Classification for research that does pose more than minimal risk for participants
