7 Cellular Respiration
Introduction: Cellular Respiration
Your body is a chemical machine; and like any machine, it uses energy to do work. The process of doing work in your body involves two essential stages: converting energy taken in as food into a usable form, and then using that energy to carry out the chemical processes that make you alive. Work includes everything your body does; from maintaining your internal temperature and regulating your heartbeat to reading this introduction, contracting muscles, digesting food and excreting wastes. Metabolism is the sum of all of the chemical processes carried out by your body (work). Metabolic rate is the rate (amount per unit of time) at which your body expends energy to do this work.
Our cells cannot use the energy in food directly. Instead, they need to convert that energy into a useable form. Adenosine triphosphate (ATP) is the energy source that all organisms use in just about every cellular process requiring energy. Our cells transfer the energy stored in organic molecules to ATP through a process called cellular respiration.
During cellular respiration, proteins called enzymes bind with substrate molecules (like the sugar glucose) and help to break the molecules apart. In doing so, the cells harvest the energy stored within chemical bonds. Through several enzyme-mediated chemical reactions, this energy is transferred and stored in molecules of ATP. When our cells need energy to do any form of work, ATP is broken down and the stored energy is released and used to fuel chemical reactions. In other words, we eat food, our cells convert food molecules to ATP, and ATP is the fuel that is burned when our cells are doing work. Without enzymes, none of this would be possible.
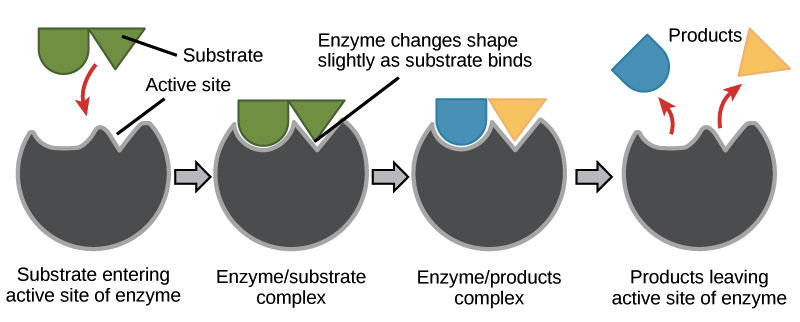
Enzymes are specific; they only bind to certain substrate molecules, and not all cells have the same enzymes. Additionally, not all organisms have the same enzymes. For example, we all know that termites eat wood. However, they cannot actually metabolize the cellulose found in plant cell walls because termites don’t produce cellulase (the necessary enzyme). Instead, microbes in a termite’s gut that have cellulase break down the cellulose into a useable form of glucose that the termite can metabolize. For another example; when a person lacks the enzyme lactase, their body cannot break down the sugar lactose, which is found in dairy products. The lactose instead passes undigested into the large intestine where bacteria metabolize it (they have lactase), producing gas and digestive discomfort. We call this condition lactose intolerance.
Aerobic respiration involves the complete oxidation (removing electrons) of organic molecules, like glucose. In this process, oxygen is the final electron acceptor in a series of enzyme-catalyzed chemical reactions. The chemical formula for aerobic respiration is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy (heat + 36 ATP)
Now, this might appear to be a simple and straightforward process. But what the simple chemical equation is missing is all the stuff that that arrow represents. So let’s take a look at what goes on behind that deceptively simple looking arrow.
Cellular Respiration: What’s really going on?
Cellular respiration starts out with glycolysis, in which glucose (or another similar sugar) is oxidized and broken into 2 product molecules (the word glycolysis actually means breaking sugar). During glycolysis, glucose is brought into the cytoplasm of a cell where it is basically attacked by enzymes that steal a couple electrons. The process of stealing or harvesting electrons is called oxidation; hence the other name for cellular respiration, oxidative metabolism. In order to accomplish this task, these enzymes use the energy from 2 molecules of ATP, and hand the stolen electrons to an electron carrier called nicotinamide adenine dinucleotide (NAD+ for short). Accepting 2 electrons reduces each NAD+ molecule into NADH (we’ll come back to these later).

After a bit of atomic rearrangement and several more steps, the glucose is split into 2 molecules of pyruvate. This whole process involves 10 chemical reactions and a bunch of enzymes, but it produces 4 ATP molecules in the process, for a net gain of 2 ATP molecules.
Next, the pyruvate molecules are brought into the intermembrane space inside the mitochondria where they are further oxidized. Removal of another electron from each pyruvate releases some carbon dioxide as waste, and 2 more NAD+ molecules are reduced to NADH. The remaining 2-carbon molecules (called an acetyl group) are combined with another coenzyme (Coenzyme A) to form 2 molecules of acetyl-CoA, which is transported across the mitochondrial inner membrane into the matrix. Once acetyl-CoA is in the matrix, the 2-carbon molecule is released and enters the Krebs cycle, and Coenzyme A returns to the intermembrane space to go get more acetyl groups.
The Krebs cycle is another really complicated process involving many enzymes and chemical reactions. But put simply, it goes like this. The 2-carbon molecule is added to a leftover 4-carbon molecule from the previous cycle to form a new, 6-carbon molecule. That 6-carbon molecule gets oxidized over and over, and several NAD+ molecules get reduced to NADH. There’s also another electron-carrying coenzyme called FAD that that gets reduced into FADH2 along the way. As electrons are harvested, CO2 is released as a waste product and another ATP gets built. At the end of the Krebs cycle, the leftover 4-carbon molecule is combined with an incoming 2-carbon molecule and the whole things happens again.
Alright, well so far we haven’t made much ATP. We only got 2 from glycolysis and 1 from each of the acetyl groups brought into the Krebs cycle. That’s only 4 ATP form a whole glucose molecule! I thought this was supposed to run all of our life processes! Remember all those NADH and FADH2 molecules? Well we just made 12 of them (10 NADH, 2 FADH2), and each one has 2 high energy electrons ready to get used.
NADH and FADH2 each carry their electrons to the third part of cellular respiration, the electron transport chain (ETC). The ETC is a series of enzymes that transport electrons from one enzyme to another in a chain. Along the way, each enzyme uses a bit of the energy from these electrons to pump free protons (also called hydrogen ions) out of the matrix and into the intermembrane space. At the end of this whole process, the electrons that were harvested all along the way are combined with an oxygen molecule and a couple free protons to form water.
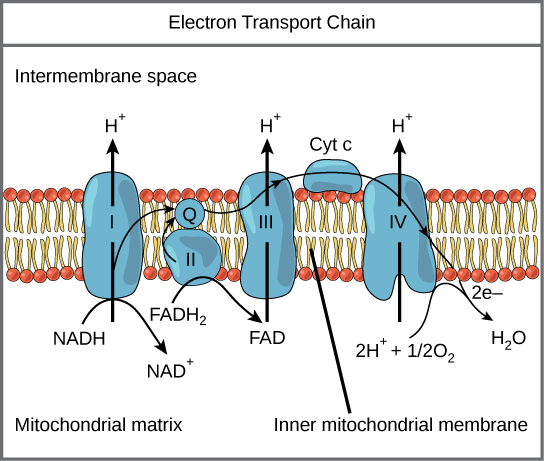
But we’re not done yet. The active transport of protons creates a concentration gradient with lots of protons outside the matrix and few protons inside the matrix. Of course, the universe doesn’t like concentration gradients and the protons are driven to equalize the disparity by re-entering the matrix. Since they are charged particles, the ions can’t pass through the inner membrane directly. Instead, they have to move through a very important enzyme called ATP synthase. This enzyme uses the movement of protons through it to attach phosphate molecules and adenosine diphosphate molecules; thus, synthesizing ATP. Most of the ATP produced during cellular respiration is synthesized by ATP synthase (on average 28-30 ATP in eukaryotes).
Wow! I can’t believe we represent that entire process with a simple arrow!
Aerobic versus Anaerobic respiration
We humans (along with all the other animals) are aerobes, which means we simply must have oxygen to survive. Without oxygen, we have no final electron acceptor for our electron transport chain, and cellular respiration shuts down. Without cellular respiration, we don’t make enough ATP and after a while, well, we die. But some organisms are able to survive without any oxygen through the process of anaerobic respiration. Just like aerobic respiration, anaerobic respiration involves the oxidation of sugars and ATP is produced. However, instead of using oxygen as the final electron acceptor, anaerobes use different molecules in its place. For example, methanogens are anaerobic prokaryotes in the Domain & Kingdom Archaea. They use carbon dioxide (CO2) as their electron acceptor at the end of the ETC and produce methane (CH4) as a product.
Fermentation is another form of anaerobic respiration, and involves the incomplete oxidation of sugars. In fermentation, glucose is broken down through glycolysis, and a small amount of ATP is produced. However, in the absence of oxygen further oxidation of pyruvate is not possible. If the organism can’t use a different substitute electron acceptor, the only way to keep producing ATP (and keep staying alive) is to keep doing glycolysis. But if there’s no molecule to take the electrons from the ETC, then the whole thing backs up and NADH get stuck holding electrons with nowhere to go. So the solution is for NADH to dump its electrons and go back to get more from the next glucose molecule, thus allowing glycolysis to continue.
There are several forms of fermentation and different critters use different forms. For example, when you exercise, you often use up ATP faster than you can take in oxygen. Under these conditions, your muscles switch from aerobic respiration to fermentation. Lucky for us, NADH is perfectly happy to give its electrons back to pyruvate. Reduction of pyruvate produces lactate, which is converted into lactic acid. Lactic acid buildup within muscle cells is a common contributor to soreness after exercise, this process allows us to produce enough ATP to stay alive until we can get oxygen back into our cells and return to aerobic respiration.
Yeasts are a type of single-celled fungi that use fermentation as their preferred form of cellular respiration. After glycolysis, pyruvate gets converted into acetaldehyde which serves as an electron acceptor for NADH. Reduction of acetaldehyde produces ethanol and carbon dioxide as products. We humans utilize this process in the production of bread and beverages such as beer and wine.
The chemical formula for ethanol fermentation is:
Suppose we wanted to measure the metabolic rate of yeasts. What products could we measure in this process?
Measuring cellular respiration
We can’t measure cellular respiration directly (e.g., how fast every cell in your body produces ATP from food molecules). However, we can measure the results of cellular respiration. For example, we can measure the amount of heat given off by an organism, the rate of consumption of chemical reactants, or rate of production of chemical products during cellular respiration. The rate of consumption or production of these materials is directly proportional to an organism’s metabolic rate. For example, the more O2 is used up, or CO2 is produced over a set amount of time, the higher the metabolic rate.
Experimental Design: Standardizing Units of Measurements
Standardizing units is one way of controlling for extraneous variables that might affect the results of an experiment. For example, in lab this week, we will be using the same volume of solutions in each treatment of our fermentation experiment. That way our estimations of metabolic rate are comparable as a rate of CO2 production from a fixed volume of yeast and carbohydrate solutions.
However, we can’t always control the amount of “subject” in an experiment; therefore, we sometimes take mathematical steps to control for a variable. For example, different organisms don’t consume energy at the same rate. Does a 3-ton elephant use the same amount of energy as a 20-gram mouse; or more/less? But is this really a fair comparison? The elephant has more cells in its body, so you would clearly expect it to consume more energy as a whole. If we want to compare metabolic rates between these organisms, we need to standardize our units of measurement. In other words, we need to find a unit of measurement that minimizes the effect (controls for the variable) of number of cells in the body. We can do this by dividing the whole-body metabolic rate of each organism by its body weight. This way we are comparing the rate of energy consumption per each unit of mass (or number of cells, assuming most cells weigh about the same). If we are using a group of animals, we simply divide the whole group metabolic rate by the weight of the group of animals. Now we have the per gram metabolic rate and we can compare these values for any organisms we want regardless of number or body size.
Doing the math
Familiarize yourself with the following equations. You will use them to estimate the metabolic rates of our organisms in Experiment 2. For each group of worms, you will calculate the whole-body (whole group) production rate of CO2 per gram of body weight. In order to have enough cells doing respiration, our experiment will use 15 mealworms at a time. Thus, the whole-body respiration rate will represent the average respiration rate per individual in each trial.
Equation for whole body respiration rate of animals:

Equation for per-gram respiration rate of animals:
 Think about it: Why is the per gram respiration rate higher than the per individual rate? Which is a better measurement to focus on?
Think about it: Why is the per gram respiration rate higher than the per individual rate? Which is a better measurement to focus on?
Experiment 1: Fermentation
We know that enzymes are essential for cellular respiration to take place, and that different enzymes help metabolize different food molecules. We also know that organisms can lack certain enzymes (e.g., lactose intolerance) causing them to not metabolize certain foods. Yeasts are single-celled fungi that metabolize sugars through fermentation and produce ethanol and carbon dioxide gas. We can estimate the yeasts’ rate of cellular respiration by measuring the amount of CO2 produced over a period of time. We will use this understanding to examine cellular respiration in yeasts and try to determine which of six carbohydrate substrates yeast can best metabolize.
Our research question for this experiment is: Which of these carbohydrates can yeast best utilize to perform cellular respiration?
We will use the following carbohydrate substrates in solution:
● 10% glucose – monosaccharide, hexagonal sugar
● 10% fructose – monosaccharide, pentagonal sugar, often called fruit-sugar
● 10% galactose – monosaccharide, similar to glucose but with slightly different shape
● 10% sucrose – disaccharide (glucose + fructose), often called table-sugar
● 10% lactose – disaccharide (galactose + glucose) component in milk.
● 1% starch – polysaccharide chain of glucose molecules
● Distilled H2O – control
Procedure:
- Obtain a CO2 sensor and a 250 mL respiration chamber.
- Press the power button on the CO2 sensor, red light will start blinking.
- Open the graphical analysis app on your phone or wireless device. Select New Experiment → Wireless Devices and select the discovered sensor that matches your sensor’s ID code. Touch Done. The sensor’s light will change to flashing green. (Be sure your app is connected to your sensor).
- Label a small respiration chamber (250 mL) with your team’s assigned carbohydrate treatment, and then add 10 mL of yeast slurry and 20 mL of the carbohydrate solution to the chamber and swirl to mix the solutions.
- Insert the CO2 sensors into the chambers and then wait 5 minutes for the yeasts to begin to metabolize the sugars.
- To set up the time for your experiment, touch Mode on the app screen. Touch End Collection, then touch Duration & set the Duration to 600 seconds. Touch Done.
- Allow at least 3 minutes for the sensor to warm up. Touch the symbol and select Meter. Record starting CO2 ppm in Table 5.1, then touch Collect to start recording data. You can see your data graph and values by tapping the symbol again and selecting graph or graph and table.
- After 10 minutes, record final CO2 ppm in Table 6.1.
- Touch the symbol in the lower left of the app screen and select Apply Curve Fit, then select Linear if not already selected.
- The graph will display a line fit over the data in the format y=mx+b, where y is CO2 concentration (ppm), x is time (s) and m=the rate of change in CO2 ppm per second.
- Multiply the m value (slope of the line) by 60 to convert the time value to minutes.
- Record the CO2 Production rate (ppm/min) in Table 5.1.
- Repeat steps 4-9 twice to obtain 3 total replicates with your carbohydrate treatment, and add your team’s data to the class data spreadsheet.
Experiment 2: Aerobic Respiration
You are all no doubt familiar with insects, those crunchy critters, that run about under our feet and occasionally fly directly into our eyeballs. What you might not be aware of is how they work. As far as basic metabolic functions go, they differ very little from animals we are more familiar with: mice, frogs, elephants, us humans. However, insects do differ in some key ways.
Insects are invertebrates, which means they lack an internal segmented backbone (spine). They are also in the Class Arthropoda (literally “jointed feet”). So, like all arthropods, instead of an internal support structure for muscles and other squishy parts to hold on to, insects rely on an external skeleton (or exoskeleton). The exoskeleton is composed primarily of chitin (carbohydrate polymer also used by fungi for cell walls) and consists of many hard plates held together by softer connecting membranes. Inside, insects have an open circulatory system. Unlike your blood, which is contained in vessels and separated from other fluids like lymph & interstitial fluids (the watery fluid between squishy parts), arthropods have hemolymph. Hemolymph is a sort of mixture between blood and other body fluids that flows openly throughout the body cavity. It is collected from the body by vessels that return it to the heart where it is pumped back into the body cavity. Instead of the hemolymph carrying oxygen to cells and carbon dioxide away, like your blood does, insects have a tracheal system for getting oxygen in and carbon dioxide out. Since they lack lungs, insects have pores in their exoskeleton that open into air-filled tubes called trachea.
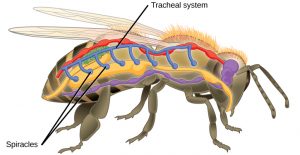
These breathing tubes pass through body tissues, and branch into smaller and smaller tubes called tracheoles . At their finest, tracheoles are so numerous that every cell in an insect’s body resides within 2-3 cells of a tracheole. Oxygen and carbon dioxide diffuse in and out of the cytoplasm directly with the air in the tracheoles.
Ok, so what does this have to do with cellular respiration? In Experiment 2 of this week’s lab, we will be using insects to assess the effects of temperature on cellular respiration rates. We will account for differences in insect body size by calculating respiration rate per gram of body mass. This way we can control for (and negate) the compounding effects of different critters having more or less cells in their bodies. We will use two temperature treatments and look for patterns in the data to help us understand how environment plays a part in regulating metabolic processes.
Since insects are animals, they harvest energy from food molecules primarily through aerobic oxidative metabolism (AKA: cellular respiration), just like you. Unlike you, insects are ectotherms. This means that their body temperature is highly influenced by the temperature of their environment. In most ectotherms, as body temperature changes there is often an observable change in cellular respiration rate. We will thus explore the effects of temperature on respiration rate in mealworms. Mealworms are often raised as food for pet animals (e.g., lizards, bats, hedgehogs), and are apparently very nutritious. They also appear to be able to eat and digest polystyrene (Styrofoam) without detrimental impacts.

Procedure:
- Obtain a CO2 sensor, a 250 mL respiration chamber, and a plastic tub.
- Weigh your respiration chamber and record its mass.
- Obtain 15 mealworms from the colony, add them to the respiration chamber, and weigh both together. Record the chamber + animal mass, and calculate the mass of your animals.
- Place the chamber in the correct treatment conditions for 10 minutes to allow the animals to come to treatment temperature. For cold treatment, bury the chamber in ice inside the tub.
- Press the power button on the CO2 sensor; the red light will start blinking.
- Open the graphical analysis app on your phone or wireless device. Select New Experiment → Wireless Devices and select the discovered sensor that matches your sensor’s ID code. Touch Done. The sensor’s light will change to flashing green. (Make sure your app is connected to your sensor).
- To set up the time for your experiment, touch Mode on the app screen. Touch End Collection, then touch Duration & set the Duration to 600 seconds. Touch Done.
- After the 10 minute “temperature adjustment,” touch the symbol and select Meter. Record starting CO2 ppm, then touch Collect to start recording data. You can see your data graph and values by tapping the symbol again and selecting graph or graph and table.
- After 10 minutes, record final CO2 ppm and calculate whole-body respiration rate, and per gram respiration rate (see above) for your mealworms/treatments.
- Repeat steps 3-9 with new mealworms to obtain 2 replicates with your treatment.
- Add your replicate data to the class spreadsheet.

